Drugs to prevent COVID-19: living guideline | WHO: As of 7 March 2023, over 759 million people worldwide have been diagnosed with COVID-19, according to estimates from the WHO dashboard (7). The pandemic has so far claimed more than 6.7 million lives. This living guideline responds to emerging evidence from randomized controlled trials (RCTs) on prophylactic interventions for COVID-19. These interventions aim to prevent the disease developing in those who are free from disease. Interventions could target whole populations, those at higher risk of becoming infected with SARS-CoV-2 due to their work, social circumstances or a particular exposure, or target those at higher risk of death and poor outcomes. As of 6 February 2023, there are 14 225 registered or ongoing trials investigating various interventions for COVID-19 (see Section 7) (8). This rapidly evolving evidence landscape requires trustworthy interpretation and expeditious clinical practice guidelines to inform clinicians, patients, governments, ministries and health administrators.
This is a living guideline from the WHO. Recommendations will be updated, and new recommendations will be added for other prophylactic interventions for COVID-19. The guideline is written, disseminated and updated in MAGICapp, with a format and structure aiming to make it user-friendly and easy to navigate (24). It accommodates dynamic updating of evidence and recommendations that focuses on what is new while keeping existing recommendations, as appropriate, within the guideline. Section 4 outlines key methodological aspects of the living guideline process including special considerations relevant to the special area of scientific evaluation of prophylactic interventions.
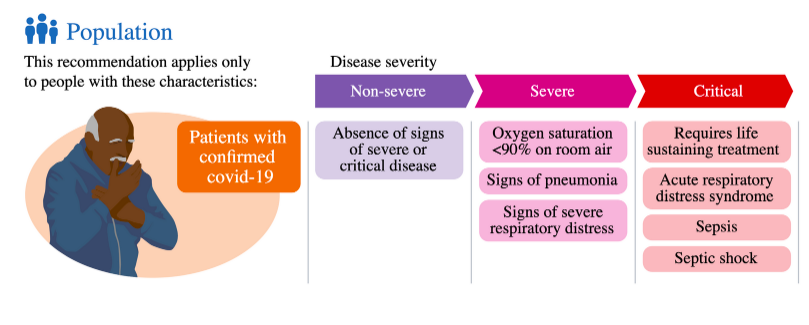
Drugs to prevent COVID-19: living guideline | WHO: While the panel unanimously supported the direction of the recommendation, there was substantial discussion on the strength of the recommendatIon for tixagevimab-cilgavimab and, ultimately, the 14 GDG members who voted for a strong recommendation were outnumbered by the 17 who supported a conditional recommendation. All members of the panel acknowledged that the available in vitro data suggests that the results of the published clinical trials are now, at best, highly uncertain. However, the panel was split regarding the certainty with which guideline users could rule out that the prevailing SARS-CoV-2 sublineages in their region would be resistant to tixagevimab-cilgavimab neutralization. Those who voted for a strong recommendation pointed out that emerging sublineages tended to rapidly replace older sublineages all over the globe and that regional real-time monitoring of circulating SARS-CoV-2 sublineages was onerous and unrealistic in most geographical areas.
Notwithstanding, those who voted for a conditional recommendation argued that, provided that these conditions could be satisfied, some highly vulnerable patients may choose to receive tixagevimab-cilgavimab. The panel inferred that restricting use of tixagevimab-cilgavimab to clinical trials would be futile considering the large number of participants required to demonstrate clinical effectiveness. Emerging evidence The unprecedented volume of planned and ongoing studies for COVID-19 interventions implies that further evidence will emerge to inform policy and practice. An overview of registered and ongoing trials for COVID-19 therapeutics and prophylaxis is available from the Infectious Diseases Data Observatory, through their living systematic review of COVID-19 clinical trial registrations, the WHO website and other repositories, such as the COVID-NMA initiative
his is a living guideline from the WHO. Recommendations will be updated, and new recommendations will be added for other prophylactic interventions for COVID-19. The guideline is written, disseminated and updated in MAGICapp here
- Anti-Tobacco Video Contest
- Health Officer |YAC | Nursing jobs latest
- Self-Care Month 2024 | WHO
- Antimicrobial resistance | A top global health threat
- Community Outreach Coordinator | Our Sansar | ngo jobs 2024
- WHO bacterial priority pathogens list, 2024 | latest WHO document
- Research Officer | TPO Nepal | ngo jobs 2024
- World Hypertension Day 2024 | Know theme
- Staff Nurse | INF Nepal | ngo jobs 2024
- Vacancy for Sr. Program Officer | NGO Jobs | SISo Nepal
- Webinar on World Hand Hygiene | Register today
- World’s First 5-in-1 vaccine against meningitis | Men5CV
bachelor jobs bph jobs health health for all health guidelines new health jobs healthjobs healthjobs in nepal health jobs vacancy health public health update ingo jobs jobs after passing bachelor jobs for bph jobs in nepal jobs in ngo ngo jobs ngo jobs vacancy ngo jobs vacancy for bph ngo job vacancy 2021 nurse jobs nurse jobs 2021 nurse vacancy nursing insurance nursing job nursing jobs nursing jobs 2021 nursing jobs in nepal nursing law nursing officer Nursing Vacancy Public health Public health concern public health important days Public health in Nepal publichealth jobs publichealthjobs public health updated Staff Nurse Staff Nurse and HA Vacancy | Nepal Army 2021 staff nurse vacancy staff nurse vacancy in ngo 2021 nepal staff nurse vacancy kathmandu who guidelines WHO official

Hey ! I am Nepal Health Magazine Bot , yet powerful enough to approve, disapprove , delete, ban anything and anyone.



