Clinical trials are research studies that evaluate new ways to improve treatments and quality of life for people with diseases like HTN,Diabetes,COVID-19,etc . Clinical trials are an option for all patients to consider and play an important role in improving healthcare. Results from clinical trials help develop new drugs, new diagnostic tools, and new clinical procedures. Clinical trials can also spur new questions for research, which lead to new discoveries over time and help us to better understand diseases like HTN,Diabetes,COVID-19,etc. All the drugs and tools we have today were made possible because patients volunteered for clinical trials.This article is intended to explain all clinical trials and It’s process in Nepal.
[lwptoc min=”4″ title=”Table of Contents”]
Every clinical trial has what are called “eligibility criteria”. For example, a patient may need to have a certain medical condition, or gender, or other specifics to meet the criteria of participating in the clinical trial. Otherwise they are not eligible to join that clinical trial and could seek another trial that better fits their situation.
Patients who want to volunteer need to understand the potential benefits are :
- Patients may personally benefit from receiving new therapies or procedures.
- They receive close supervision from clinical staff.
- Sometimes the cost of the new drugs or testing is free or waived.
- Participants may also be part of a research breakthrough that helps thousands of future patients.
Patients face the risk including :
- Patient may not benefit from the new treatment.
- Additionally, participating in a clinical trial requires commitment. Trials can be lengthy or demanding, and may require extra treatments, extra time in the clinic, or tests or costs that are not part of standard care.
- It is possible, if the study is randomized, that they may be part of the control group that does not receive the new drug being studied.
Table of Contents
Informed consent
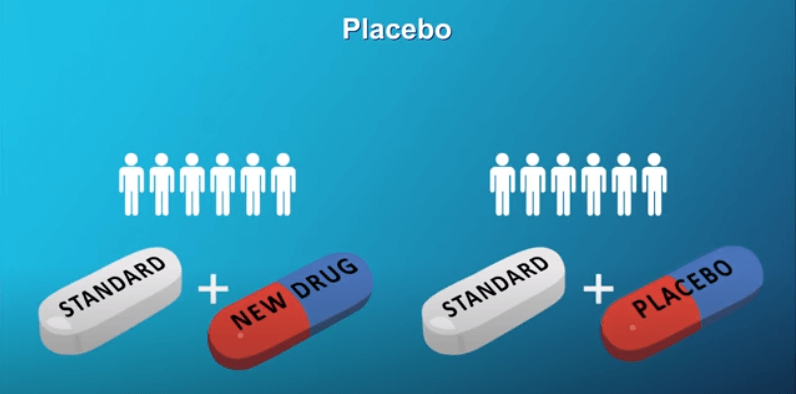
“Informed consent” is a process that helps patients make an informed decision about whether to join a clinical trial. An “informed consent document” will be provided that explains the purpose, procedures, and potential benefits and risks of the trial. There will also be thorough discussions between the doctor and patient so that all questions can be clearly answered. In late-stage clinical trials, participants are generally split into 2 groups at the beginning of the study. One group receives the standard treatment plus the new treatment. This group is called the “Treatment Group”. The other group receives the standard treatment only, and does not receive the new treatment. This group is known as the “Control Group”. A computer is used to randomly assign the patient to either the Treatment Group or the Control Group. Randomization helps to prevent bias because neither the patient nor the doctor can choose which treatment group a patient is assigned to. Some clinical trials may want to study the effectiveness of adding a new treatment to a standard treatment to see what works better. In these studies, half the patients will receive the standard treatment plus the new treatment, and the other half will receive the standard treatment plus a “placebo”. A “placebo” is a treatment that looks identical to the new treatment, but contains no active ingredient.
Blinding in Clinical Trials
These types of studies are often called “blinded“, meaning that one or more parties involved in the trial do not know which patients have been assigned to which treatment. “Single-blinded” means that only one party (either the researchers or the patients) knows which patients have been assigned to which treatment. “Double-blinded” means that both the researchers and the patients do not know which patients have been assigned to which treatment. The strongest, most reliable results come from clinical trials in which the treatment is double-blinded, the patients are randomized, and the study has a control group. This is called a “double-blinded, randomized, controlled clinical trial” and is the most effective, reliable and scientific clinical trials. Clinical trials are carefully monitored to protect patients, and several layers of review committees are involved to protect patients from any possible harm. Patients can leave a clinical trial at any stage. However, they should discuss this with their doctor before exiting, to ensure the safest procedures are followed.
Phases of Clinical Trials
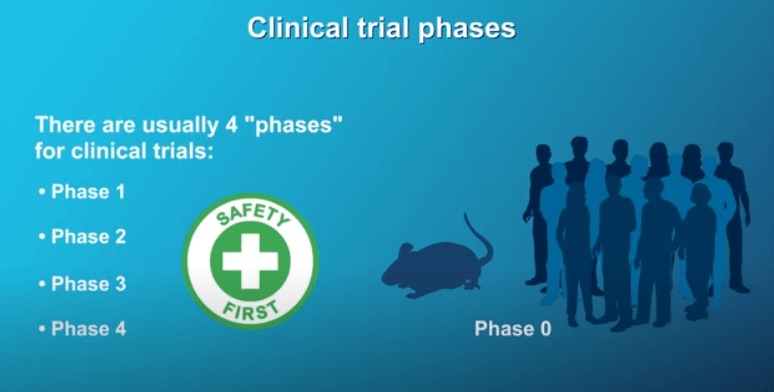
There are 4 different phases of clinical trials. Safety is emphasized in each phase.
If a trial does not have a positive or safe outcome in Phase 1, then it will not be allowed to proceed to a Phase 2 trial, and so on. Before a Phase 1 trial begins, there is a Phase 0, where the new treatment has already been found to be safe in animals or healthy human volunteers. In Phase 1 clinical trials, a new treatment is tested in a small group of participants for the first time to determine a safe dose and see how the treatment affects the body. Phase 2 is conducted with a larger patient group than the previous phase and studies how well a new treatment or combination of treatments works for a certain type of cancer, like pancreatic cancer. At this stage the eligibility criteria become more restrictive. Phase 2 studies can be either randomized or non-randomized. Phase 3 trials are conducted in an even larger group of participants. This phase involves a randomized design, comparing the new treatment to the standard treatment. If a Phase 3 clinical trial is positive, typically the new treatment will be approved and licensed for use in the United States by the Food and Drug Administration (or FDA). After the treatment has been approved, Phase 4 clinical trials involve monitoring to continue to assess safety, effectiveness, and optimal use.
Clinical trials play an important role in moving science forward and can be a hopeful option for many patients with HTN,Diabetes,COVID-19,cancers and new outbreak.
Clinical Trials in Nepal
Nepal Health Research Council is only the official and responsible body responsible for trial registry in Nepal. A trial registry is defined as an organization or website that either: lists clinical trials being conducted (or that have recently been conducted) provides a mechanism for patients or others to register their interest in participating in a clinical trial provides a link between potential participants and clinical trials. NHRC register community trials and randomized controlled trials of registered medical devices
Requirement for Clinical Trials in Nepal
WHO/ICTRP mandate to establish a trial registry at the conveniences places for researchers. [Note: Drug Act 2035 mentioned any person who intends to carry out a clinical trial of any new drug shall obtain a license from the department, as prescribed, for such trial. For conducting the clinical trial in Nepal, medicine registration guidance (Issued under Drug Registration Regulation 2038) section 2.18 mentions an approval of Nepal Health Research Council (NHRC) and other government-approved agencies are required.] NHRC welcomes all the Worldwide researchers,Industry/Company Researcher and NGO/GO’s researcher for clinical trial registry and needs following document submissions to initiate trial registry.
- Trial Protocol (Proposal)
- Approved ethical clearance letter
- Agreement letter from study site
- Researcher profile
Monitoring of Clinical Trials in Nepal ?
All clinical trial will be independently monitored by NHRC , MoHP and WHO/ICTRP . All clinical trials registered in NPCTR will comply with and be functional according to the policy and mandate of WHO/ICTRP Will be a primary registry.All registries are the same but it will be in Nepal.They will get the NPCTR number and that number will be equivalent to WHO/ICTRP. Researchers will publish their manuscript at any PubMed index journal or journal of ICMJE mandate group.All registries are the same but it will be in Nepal.
NHRC have developed portal NPCTR and receives online submission for trial registry. Currently six trial registry have been registered and are listed below :
- Effect of peer based support for improving access to family planning services among HIV positive women and adolescents in remote villages of far western province of Nepal- a cluster randomized controlled trial
- Efficacy of Favipiravir in treatment of mild & moderate COVID-19 infection in Nepal: a multi-center, randomized, open-labelled, phase III clinical trial
- Comparison of different doses of morphine added to hyperbaric bupivacaine in patients undergoing lower abdominal gynaecological procedure under spinal anaesthesia conducted at BPKIHS, Dharan
- Effectiveness of traditional Ayurvedic formulation in clinical improvement of COVID-19 patients – randomized, two-armed, open-label, pragmatic trial in COVID Hospitals in Nepal – a pilot study
- To find out the impact of adaptive radiotherapy planning on tumor coverage and normal tissues for head and neck cancers
- Comparison of ketofol and propofol for Electroconvulsive therapy.
This list can be found and accessed at public domain https://npctr.nhrc.gov.np/trials

Hey there, I am Nirdesh Baral, founder of Nepal Health Magazine. I am a Tech geek by passion , Public health practitioner by profession and an Ailurophile by heart and a patriot by birth




